
Executive Summary
Children’s Hospital of Orange County (CHOC) maintains a focus on patient safety for infant feeding and enteral feedings including identification of “near misses” by way of a dedicated process and team. This team employs specific technologies and tools that go beyond our initial work with human milk (HM) safety. The emphasis since CHOC’s HIMSS Davies Award for infant feeding safety in 2016 has been to sustain the success of the human milk scanning program and advance the human milk fortifier and formula scanning aspects with the use of additional people, processes, and technologies.
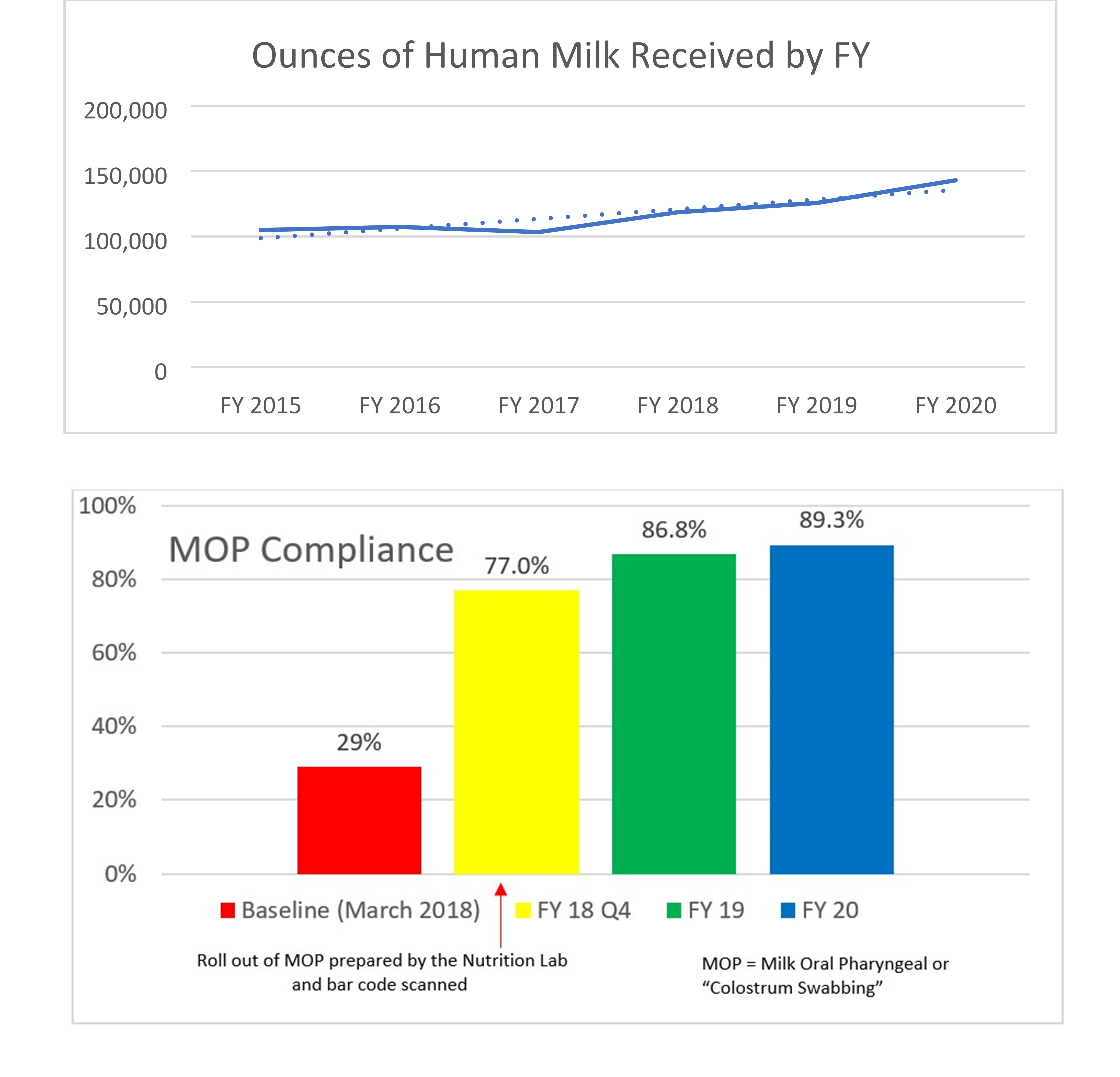
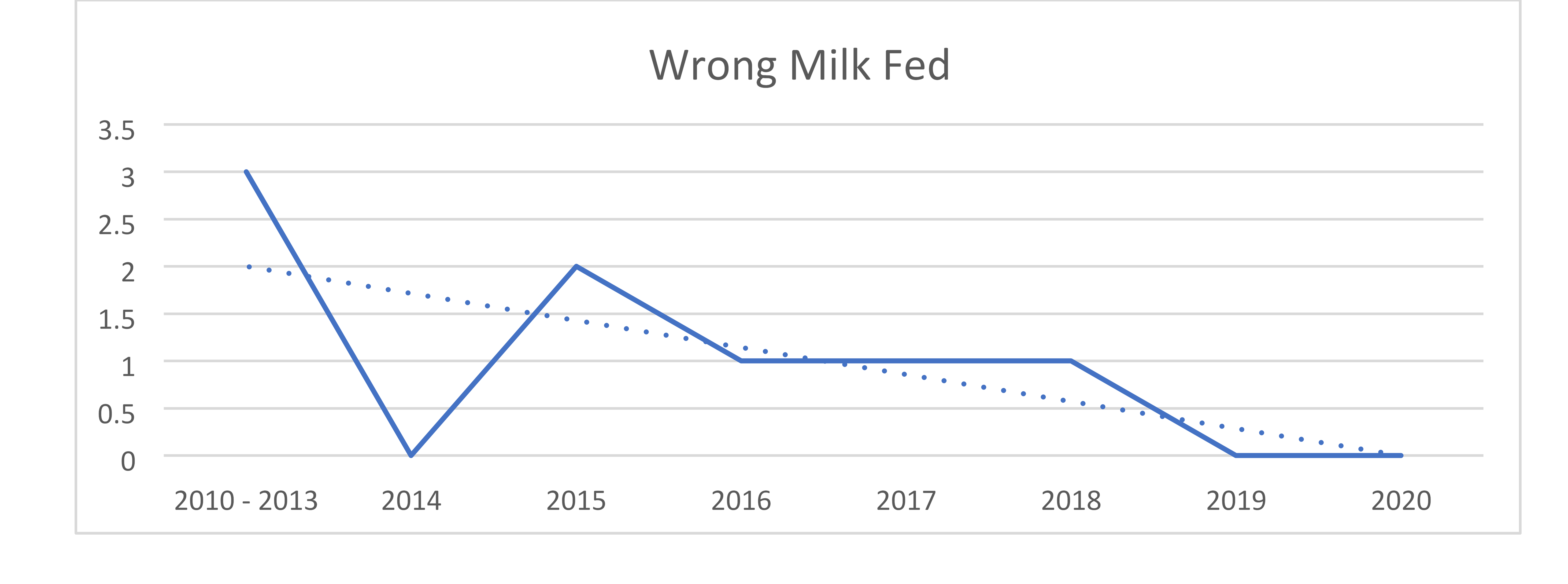
In January 2018, the organization added a formula tracking tool to scan fortifiers and formulas within the centralized preparation room known as the Nutrition Lab. Upon going live, the system was used to calculate all HM and facility-prepared formula recipes and to scan all HM additives, formulas and modulars to ensure the products match the electronic order. The system also records lot numbers and expiration dates. The near-miss data for both HM and formulas shows that, despite handling more HM and preparing more formulas each year, the number of near-misses continues to decline. The decline suggests that not only does the system itself prevent errors but also serves to provide ongoing education and assist in behavior change of the users. The more users are focused on the frequency and potential risks of such errors, the more aware and careful they be come with their own actions. The organization’s targets for infant feeding safety include: A dedicated preparation location that supports aseptic technique, specific equipment and supplies, dedicated staffing and staff hygiene, appropriate HM and formula storage, and use of technology (including bar code scanning) to improve safety and workflows. Expressed Human Milk (EHM) volumes have increased 36% since fiscal year (FY) 2015, compliance with Milk Oral Pharyngeal (MOP) has increased from 29% in FY 2018 to a current 89.3% at the conclusion of FY 2020, and incorrect milk fed prior to the changes in 2012 decreased from three to zero in FY 2019 and remains at zero through FY 2020.
Over time the key lessons learned include:
- Demonstrating the importance of sharing the near-miss data with the bedside staff as a driver of best practice, as ~80% of wrong scans were at the bedside
- Continuing education of the technology is important to ensure ongoing compliance
- Maintaining accurate integration and workflows within the EHR ensure safety and efficiency
This sophisticated combination of people, process and technology promotes a safe workflow and ensures the right child receives the right feeding at the right time.
Defining the Clinical Problem and Pre-Implementation Performance
According to the Centers for Disease Control (CDC), breastfeeding is the best source of nutrition for most infants. It can also reduce the risk for some short- and long-term health conditions for both infants and mothers. Meeting the standard to increase the number of breast-fed babies is only part of the process embraced by CHOC. The increase in the utilization of HM and infant feeding preparation brings the possibility of contamination, misadministration, and preparation inaccuracies. The feeding of HM to preterm infants is typically much more complicated than the mere act of breastfeeding(3, 4). The limited oral feeding skills of many preterm infants often results in HM being administered via an enteral feeding tube(4). In addition, fortification is commonly required to promote optimal growth and development—particularly in the smallest of preterm infants(2, 4, 5).
A mother’s own milk must consequently be pumped, labeled and transported to the hospital. It is then stored, tracked for appropriate expiration dates and times, thawed (if previously frozen), fortified and administered to the infant with care taken at each step of the process to avoid microbial contamination, misadministration (the wrong milk for the wrong patient), fortification errors and waste(1–5).
Furthermore, the use of pasteurized donor human milk (DHM) for preterm infants—when a mother’s own milk is not available—has been endorsed by many organizations(1). Therefore, appropriate procurement, storage, thawing (if received frozen), fortification, labeling and administration must occur with the same considerations of preventing contamination and fortification errors while ensuring the correctly prepared final product reaches the correct patient(1). Many professional organizations have published best practices to provide hospitals with guidelines for the safe and accurate handling and preparation of EHM and DHM infant feedings for preterm infants(1–5). These best practices emphasize the importance of preparation location, trained staff, proper identification of HM to prevent misadministration, and strategies to prevent fortification errors(1–6).
The purpose of this case study is to summarize current published best practices for the handling of HM for infants within the hospital setting(1–6) and the safe practices of managing infant formula from the time it enters the hospital until the time it is fed. Emphasis will focus on the use of aseptic technique with proper sanitation and holding times/temperatures to limit microbial growth; use of technology to prevent misadministration of HM and fortification errors as well as for tracking of expiration dates/times and lot numbers; and workflow strategies to promote safety while improving efficiencies(1–7). CHOC submitted a Davies Award-winning case study in 2016 based on the implementation of a centralized human milk preparation process. This included barcoding to effectively eliminate feedings of the wrong human milk. The organization has maintained this high reliability in error prevention, and in 2018 introduced tracking technology for all human milk fortifiers, modulars, infant formulas, and enteral formulas (taken orally or via tube feeding). When preparing HM or formula infant feedings, every HM bottle and/or product is scanned to confirm the product matches the patient and the electronic order as well as to ensure the expiration dates for HM and products and lot numbers for products are recorded. Ready-to-feed (RTF) enteral formulas and oral supplements are also scanned to confirm they match the electronic order, their lot numbers and expiration dates are recorded, and a patient-specific label is generated.
Many professional organizations, including the Academy of Nutrition and Dietetics, American Society for Parenteral and Enteral Nutrition (ASPEN), the National Association of Neonatal Nurses (NANN), and Human Milk Banking Association of North America, have published best practices to provide hospitals with guidelines for the safe and accurate handling and preparation HM and formula feedings within the hospital setting(1–5). These best practices emphasize the importance of preparation location, trained staff, proper identification of human milk to prevent misadministration, and strategies to prevent fortification and formula errors(1–6). By following these industry standards and expanding upon those best practices including the implementation of the formula tracking solution, the organization has been able to realize additional improvements such as: An increase in the ounces of HM in 2015 from 104,977 ounces to 142,903 ounces in FY 2020, compliance with MOP has increased from 29% in FY 2018 to a current 89.3% at the conclusion of FY 2020, and incorrect milk fed prior to the changes in FY 2012 decreased from 3 to 0 to date at the end of FY 2020. Patients included in the data (denominator) are all patients who received expressed human milk, donor human milk, fortifiers, formulas, and modulars. The goal is to sustain safe feeding practices for infants and remain a leader in the industry while sharing our blueprint for safe infant feeding practices. A bibliography of our published work is included in the appendix.
Designing and Implementing Model Practices and Governance
CHOC is no stranger to the safety aspects of infant feedings and enteral feedings. The original process began in 2013 with the decision to purchase a human milk bar code scanning system. This project was led by the director of clinical nutrition & lactation and an information technology (IT) project manager—also including a variety of clinical and IT team members. The success of the initial project was instrumental in the organization’s advancement to HIMSS EMRAM Level 7 and a HIMSS Davies Award in 2016.
The clinical nutrition team at CHOC didn’t stop with that process. They continued the growth and sustainment of the initial work and implemented additional technologies to improve the safe scanning of both HM and infant enteral formulas.
The organization’s lactation process improvement structure is led by the director of clinical nutrition & lactation and is co-chaired by the lactation medical director. This multidisciplinary, data-driven team works to ensure that the patient safety process has continued for nearly a decade.
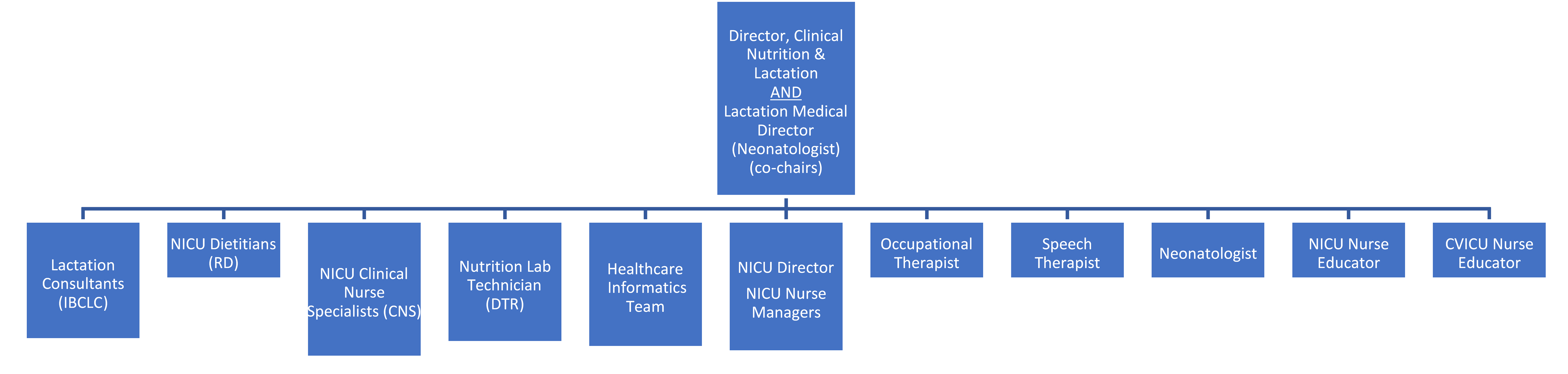
Source: Children’s Hospital of Orange County
The team followed CHOC’s governance standards for the purchase of the formula tracking solution to enhance the human milk barcode scanning process. The organization’s continued journey led to the enhancement of its existing program with the addition of the formula tracking solution in 2018. Lessons kept safety at the forefront of their decision.
Key decision requirements included:
- Must improve patient safety
- Eliminate identification, preparation and infant feeding errors
- Track expiring HM, DHM and formula to prevent expired feedings from being administered
- Identify all feeding containers and storage locations
- Increase staff productivity and satisfaction
- Integration with CPOE, ADT and EMR systems to track product information, lot numbers and expiration dates on individual infant feedings
- Calculate recipes for facility-prepared HM, DHM and formula feedings
- Reduce costs
- Track product usage and inventory to prevent waste from expiring products or excessive costs for requiring overnight delivery
- Minimize/eliminate injury and associated costs
- Reduce staffing needed to manually perform two-person verification of HM, inventory tracking, recording of lot numbers and calculation of recipes
Enabling Clinical Transformation Through Information and Technology
Clinical transformation for the safety of infant feeding encompasses people, process, technology, and physical design considerations for location and equipment.
Location
For handling of human milk, fortifiers and feeding systems, preparation location and practices that minimize microbial growth (such as adherence to good hand-hygiene practices and use of “no touch” preparation and administration techniques) are critical(1). A dedicated location for the purpose of handling human milk and formula feedings, separate from patient care areas, reduces risk of contamination and is considered a best practice(1, 2, 8, 9). EHM/DHM and formula feedings should not be prepared in any patient care area, including the patient’s bedside, due to risk of contamination(1, 2, 5, 7, 9).
Equipment and Supplies
Sinks and Dishwashers
The preparation area should contain a handwashing sink with hands-free controls(1). Unless all preparation items are disposable, a three-compartment sink or commercial dishwasher is needed to ensure proper cleaning and sanitizing of all reusable items(1, 10, 11). The dishwasher should reach a wash temperature of 66◦C (150◦F) and a rinse temperature of 82◦C (180◦F)(10, 11).
Refrigerators and Freezers
Although not required, dedicated human milk refrigerators and freezers are preferred. Adequate space to store human milk and prepared formulas that allows for appropriate airflow is important to ensure proper temperatures. Refrigeration guidelines for the storage of human milk for healthy infants at home have been described(12). Within the healthcare setting, refrigerators for human milk storage must be able to maintain temperatures between 2–4◦C (35–39◦F). Freezers must allow for temperatures at or below −20◦C (−4◦F) for long-term storage(1, 13). A reliable method of temperature monitoring is imperative to prevent loss and promote safety(1). Use of automated systems that trigger alarms when temperatures exceed desired ranges may be beneficial. Refrigeration units located in areas with limited access may help prevent tampering and waste.
Laminar Flow Hoods
While laminar flow hoods provide an additional barrier against contaminants, they are typically used in the preparation of sterile products (including medications and processing/packaging of pasteurized donor human milk)(1, 14). However, use of a flow hood does not result in a sterile finished product when used during the preparation of nonsterile infant feedings (such as mother’s own HM or facility-prepared formulas)(1, 15).
Furthermore, use of a flow hood should not be a replacement for good handing practices and aseptic technique.
Measuring/Mixing
All preparation and storage items should be made of stainless steel or food-grade plastic that is free of bisphenol A (BPA) and Di (2-ethylhexyl) phthalate (DEHP)(11). Glass items (such as graduated cylinders or beakers) are not generally used for routine handling of human milk and formula in the healthcare setting. This is due to the risk of exposure to glass particles in the event the glass cracks or breaks(11). Single-use, disposable items are often selected for human milk collection and infant feeding preparation due to their convenience and sanitation. Such items may be sterile or non-sterile as there is no evidence that non-sterile items result in higher bacterial loads in collected human milk or prepared feedings(1, 16). If reusable items are selected, they must be cleaned and appropriately sanitized between each use to prevent cross contamination. Human milk and other liquid ingredients should be measured using containers with precise graduations such as graduated cylinders, beakers, liquid measuring cups, or syringes(1). Powdered fortifiers, formulas, and additives should be measured on a gram scale that is accurate to one-tenth of a gram(1). Scales should undergo regular calibration to ensure accuracy and promote safety(1).
Staffing and Staff Hygiene
A staff dedicated to the handling and preparation of human milk and formula feedings within the healthcare setting is considered a best practice and has been shown to reduce risk of misadministration errors(1–3, 5). The staff should be trained in aseptic technique and demonstrate proper steps for handling human milk fortifiers and formulas. Hand hygiene is critical and prevents the introduction of exogenous microbial contamination to prepared infant feedings(17, 18). Disposable gowns and other personal protective items—including a bonnet or hairnet and gloves—are recommended(1). Artificial nails and long natural nails have been associated with a Pseudomonas aeruginosa outbreak in a neonatal intensive care unit(18, 19). Therefore, it is recommended that staff nails should be short, neatly groomed and unpolished(17–21).
Human Milk Storage
Stored milk should be rotated using first-in-first-out principles, using the oldest milk first. Storage times and temperatures impact nutritional quality, biologically active components in human milk, and rate/incidence of microbial growth(12, 22–26). Within the acute care setting when human milk is used for immunocompromised patients, storage recommendations are more conservative than for the healthy infant at home(1, 12).
Therefore, it is generally recommended(1, 13, 16, 22, 27):
- Fresh milk be stored in the refrigerator (≤4◦C or ≤39◦F) for a maximum of 48 hours
- Thawed unpasteurized milk be stored in the refrigerator (≤4◦C or ≤39◦F) for a maximum of 24 hours
- Thawed pasteurized DHM be stored in the refrigerator (≤4◦C or ≤39◦F) for a maximum of 48 hours
- Fortified milk be stored in the refrigerator (≤4◦C or ≤39◦F) for a maximum of 24 hours
- Hang time for continuous feedings at room temperature for a maximum of 4 hours
- Frozen human milk be stored in the freezer for six to 12 months at ≤−20◦C (≤−4◦F) or beyond 12 months at −70 to −80◦C (−94 to −112◦F)
Preparing and Administrating Human Milk and Formula Feedings in the Healthcare Setting
The handling of human milk and formulas and preparation of individual feedings within the healthcare setting requires strict adherence to guidelines. This ensures the preservation of nutrients and bioactive compounds while reducing risk of harmful microbial growth(1). Preparation accuracy is imperative to prevent feeding intolerance and promote optimal health and growth.
Steps for infant feeding preparation within the acute care setting are outlined in Table 1(1, 13, 28–32). Sterile liquid formulas and fortifiers and additives are preferred over powdered products (which are not sterile) to reduce the risk of microbial contamination. Sterile options should be used whenever possible(1, 13).
The optimal length of time between the preparation and feeding of fortified human milk and facility prepared formulas is currently unknown(13). Research has shown that over time, the osmolality of fortified human milk increases (by up to 4%) and the size of milk fat globules may become altered (possibly impacting fat digestion)(33). While shortening the storage time for fortified human milk may be advantageous, there is not enough published evidence to suggest a revision to the current recommendations for a maximum of 24 hours(1, 13).
Centralized preparation of HM and formula feedings is a best practice and has been shown to improve patient safety(1–8, 13). However, centralized handling processes often preclude the ability to prepare each individual feeding immediately prior to use. Based on current evidence, the benefits of centralized handling appear to outweigh the risks of potential changes to human milk when feedings are prepared in advance(1–8, 13, 34, 35). Facilities may want to consider the shortest amount of time realistically feasible while still utilizing centralized handling processes. To this end, some organizations have opted to prepare 12-h volumes instead of 24-h volumes, which may be beneficial to more quickly implementing feeding order changes and preventing waste(1).
In addition to safe handling practices, processes must be in place to ensure the safe administration of human milk and formulas to prevent inadvertent infusion via intravenous (IV) lines(1, 13). Enteral feeding misconnections, which can result in death, have been reported in the literature(36). The International Standards Organization (ISO) has set a standard for enteral devices requiring a female (administration set or syringe) to male (feeding tube) orientation known as ISO 80369-3(37). Feeding connection sets with this unique configuration are known as ENFit® systems(1, 37). Adoption of ENFit® compatible connectors for all enteral infusions promotes patient safety by preventing enteral feedings from being accidentally connected to IV lines or other medical device ports(1, 13, 37).
Using Bar Code Scanning Technology to Improve Safety
Bar code scanning technology is commonly used in the healthcare setting to promote patient safety by reducing the risk of misadministration (providing the wrong product to the wrong patient) for processes such as medication, blood, and human milk/formula administration(3–5). Bar code scanning is often used in lieu of a two-person double check to reduce the risk of human error and confirmation bias which may occur when a manual check is used(3–5). Such systems have been shown to reduce errors and improve efficiencies(3–5).
Scanning technology can assist with monitoring expiration dates and times. Human milk and formulas that are beyond their expiration are at greater risk for excessive microbial growth which could be particularly devastating in the critically ill infant or child. Consequently, scanning systems may add a layer of patient safety by alerting the clinician of attempts to use an expired feeding. Some systems also offer the ability to automate feeding recipe calculations and scan formulas, fortifiers, or additives to reduce risk of fortification errors(3–5). Automatically tracking lot numbers for pasteurized DHM and formulas, fortifiers, or additives is more efficient than having staff members track such information manually and provides a faster method of identifying exactly which patients received a particular product in the event of a product recall. Bar code scanning technology with human milk/formula preparation and feeding is considered a best practice and is endorsed by many organizations(1–5).
Human milk in the healthcare setting, particularly the neonatal intensive care unit, is often viewed as “medicine” or an adjunct therapy. Some of the most fragile patients are those premature or critically ill infants receiving human milk feedings. Therefore, every precaution must be taken with human milk handling to ensure safety. Aseptic technique with proper sanitation and holding times/temperatures to limit microbial growth; use of technology to prevent misadministration of human milk and fortification errors as well as for tracking of expiration dates/times and lot numbers; and workflow strategies to promote safety while improving efficiencies are worthy endeavors of all facilities (1–7). The below workflow represents HM handling and administration:
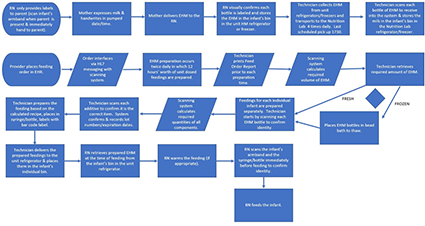
Source: Children’s Hospital of Orange County
Inbound and outbound HL7 interfaces drive the technology between the EHR and the human milk management solution.

Source: Children’s Hospital of Orange County

Source: Children’s Hospital of Orange County
Overall accuracy is dependent upon electronic orders, calculations, measurements, scanning and clinical decision support. Education takes place in a nutrition lab and classroom setting for hands on training. This ensures the handling of feeds and additives are compliant with proper aseptic technique, understanding of equipment and processes, and the utilization of the human milk management technology is complete.
Improving Adherence to the Standard of Care
The Nutrition Lab at Children’s Hospital of Orange County is responsible for preparing human milk and specialized formulas for patients with unique infant feeding needs. This process takes place in a sanitary environment and uses a special barcoding inventory system to ensure accuracy. The Nutrition Lab houses equipment specific to the needs of human milk and formula management and is staffed with registered dietetic technicians. The organization has gained international, national and statewide recognition for its human milk and handling process, which has proven to be safer for patients and more efficient for physicians and staff. It continues to see year over year improvements since the inception of the Infant and Enteral Feeding Safety initiative. Expressed Human Milk (EHM) volumes have increased 36% since FY 2015; compliance with MOP has increased from 29% in FY 2018 to a current 89.3% at the conclusion of FY 2020; and incorrect milk fed prior to the changes in FY 2012 decreased from 3 to 0 in FY 2019—and remains at 0 through FY 2020.
Source: Children’s Hospital of Orange County
Benchmark data and quality measures are not common in the breastfeeding space for very low birthweight infants (VLBW), however the organization does measure and monitor the experience of other organizations.
California Perinatal Quality Care Collaborative (CPQCC)
Calendar year 2005-2018
- The organization had breastmilk rates at discharge at 79.8% (includes all gestational ages)
- CPQCC average was 74.9%
Healthy People 2020 Goal
- Babies who have ever received breastmilk: Target = 81.9%
- CHOC is >85%
U.S. News and World Report
- Breastmilk available at hospital discharge—Threshold 75%, CHOC is consistently > 75% with an organizational goal of 80%. The organization has fallen below the organizational goal (finishing FY 2020 at 79%) but remains above the benchmark threshold.
Vermont Oxford Network
- Current stats show that 51% of North American neonatal intensive care unit discharges go home on at least some breastmilk, and CHOC remains >75%.
Analytics are available via the human milk/formula management system and the EHR. Data is managed through the clinical nutrition and lactation services, quality department, and data science department. ** Data limitations exist for statistical control due to reporting of data by year which limits data points.
Improving Patient Outcomes
Confirming proper identity of HM is only one aspect of the Infant and Enteral Feeding Safety program at CHOC. This step is critical prior to combining bottles, feeding, or discharging HM home to ensure the correct milk is going to the correct infant. The organization accomplished this confirmation with the use of a bar code scanning system in place since 2014. In addition to minimizing HM misadministration, it also began scanning fortifiers, modulars, and formulas in 2018 to reduce other errors and improve efficiencies by:
- Preventing fortifier/additive misadministration
- Monitoring expiration dates/times of prepared infant feedings as well as stored HM and fortifiers/additives
- Tracking lot numbers of DHM and fortifiers in case of a recall
- Automatically calculating fortified human milk and formula recipes
- Reducing staff time needed for two-person verification, lot tracking and recipe calculation
- Allowing for integration with the EHR to reduce risk of human error associated with manual entry
- The Infant and Enteral Feeding Safety program enhances the HM feeding process and helps to keep CHOC above the benchmark threshold for HM at discharge
Achieved outcomes since FY 2014 include:
- Reduced events of wrong milk being fed
- Prevented 3,809 infant feeding errors
- Collected 702,523 ounces of expressed milk
- Increased the compliance of administering MOP (Milk Oral Pharyngeal or “Colostrum Swabbing”) from 29% to 89.3% from FY 2018 to 2020
Available data points to support the improved outcomes include:
Source: Children’s Hospital of Orange County
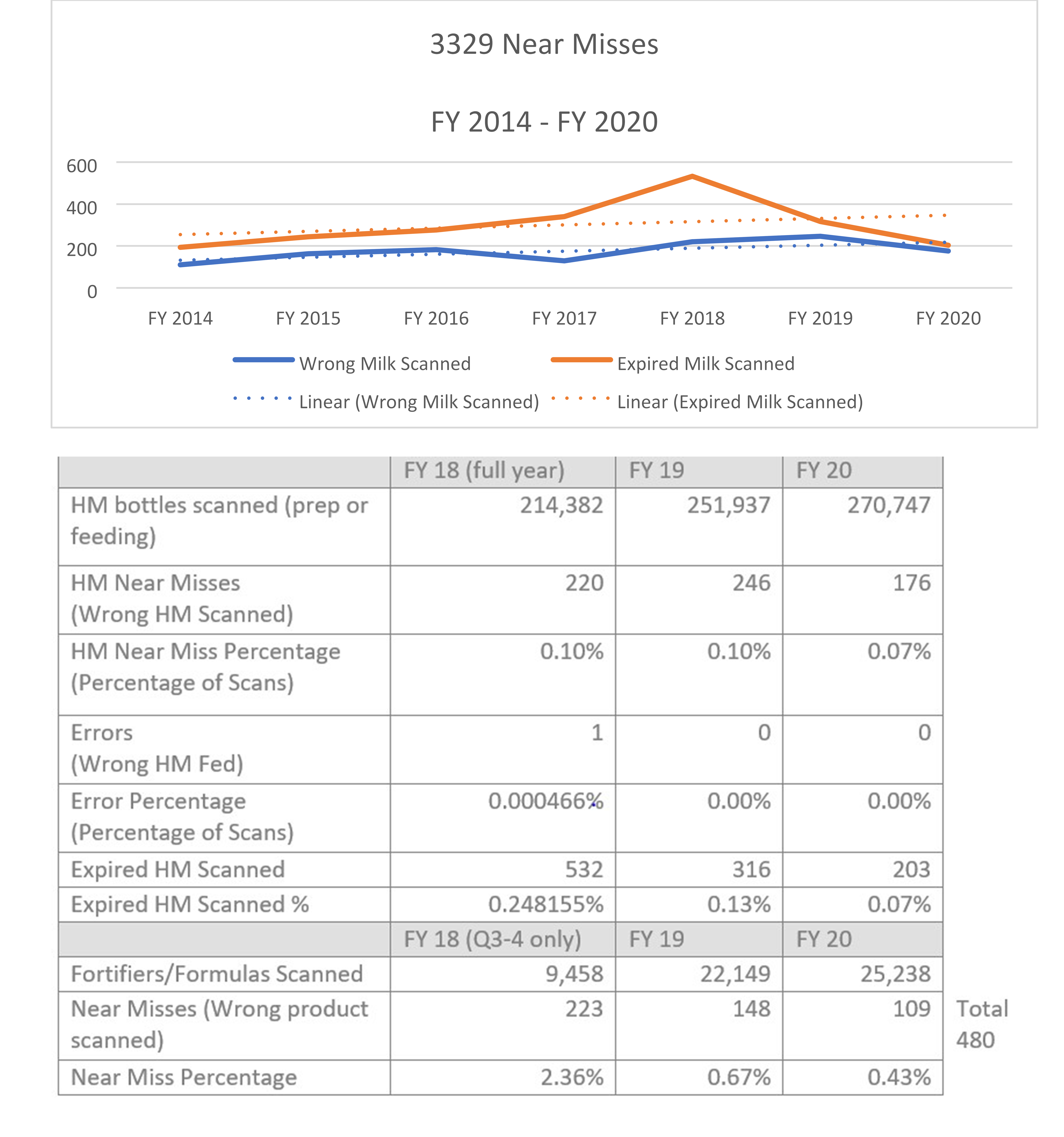
Source: Children’s Hospital of Orange County
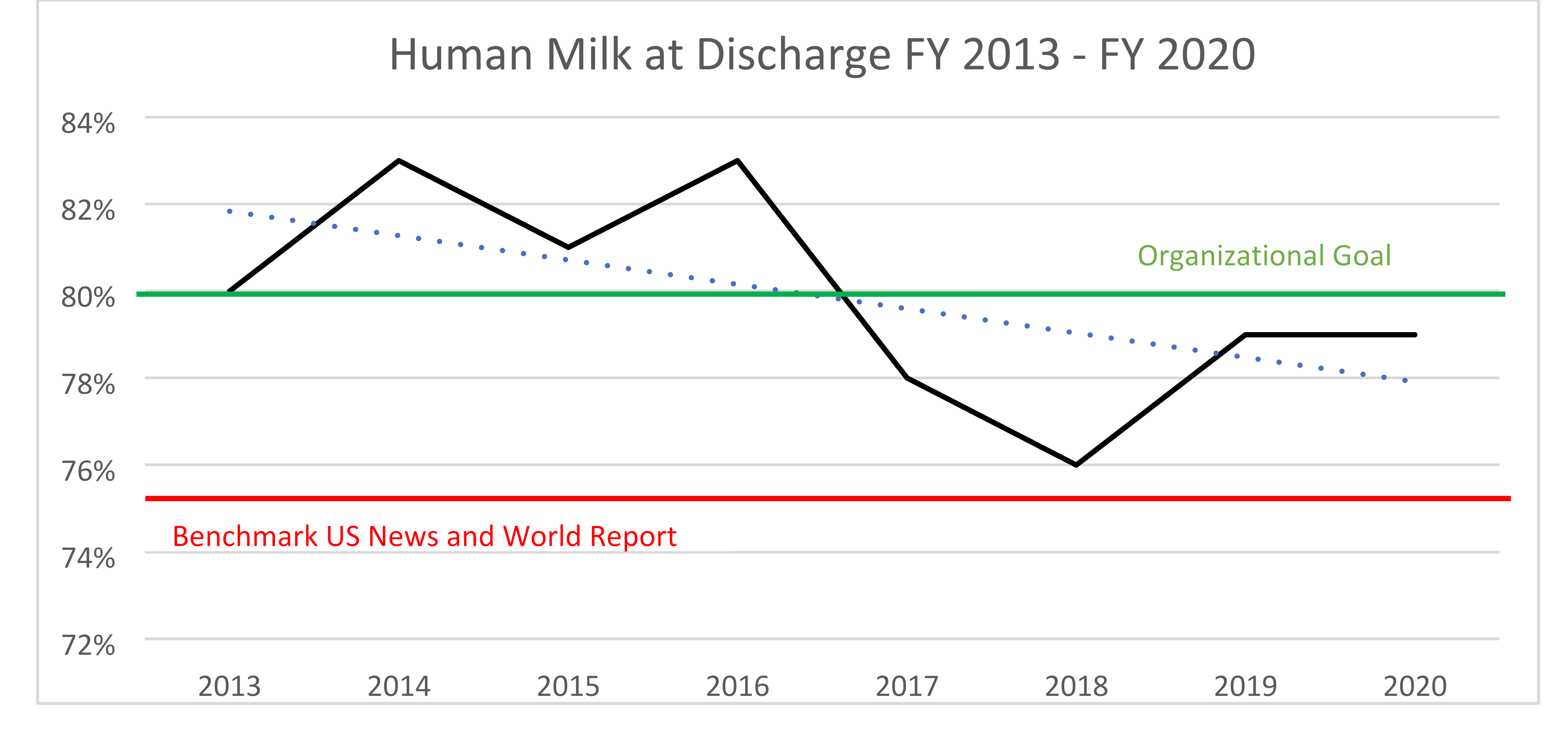
Source: Children’s Hospital of Orange County
Accountability and Driving Resilient Care Redesign
In June 2011, CHOC’s Joint Leadership Committee formed a process improvement team, including the physician co-chair and representatives from each inpatient nursing unit, clinical nutrition and lactation, quality, and transport. The team reviewed data from May 2010-December 2012 and identified 282 potential points where the existing process could fail. Scoring each item with a “risk priority number” (accounting for severity, likely occurrence, and ability to detect), the team identified the top 85 which all had scores of 160 or higher. These top 85 potential failure points were then addressed in a complete redesign of the system. In January 2013, the team moved milk preparation to a centralized location. The existing formula room was repurposed to accommodate both formula and human milk preparation. Referred to as the Nutrition Lab, it houses human milk and formula management equipment and is staffed with registered dietetic technicians.
The organization then secured a $190,000 grant from UniHealth to purchase a human milk tracking system. The software uses unique barcode identifiers to ensure babies are matched with the correct milk, and that the milk has not expired. The system launched in November 2013. Nurses previously fortified and prepared milk themselves at the bedside—a contamination risk—and then had to ask a second nurse to verify the accuracy of it at every step. That accuracy check is now done automatically. In the first three months of the barcode program, 19,989 human milk feedings were prepared by the Nutrition Lab, with 100% compliance to scanning in the lab and 99% compliance on the inpatient nursing units. In the first fiscal year, the software identified 110 near misses where the wrong milk would have been given to the wrong patient, and 193 near misses when expired human milk would have been given. CHOC has made consistent improvements over time and in 2018 added technology to track formula and fortifiers as well. In April 2019, it added functionality to be able to scan formula samples dispensed to both hospital inpatients and outpatients to ensure expired products were not inadvertently distributed and so that lot numbers would be recorded in the event of a recall. In September 2019, that process proved helpful when not only a formula product was recalled, but a representative of the U.S. Food and Drug Administration arrived and asked to see how the recall had been managed. Because of scanning, the organization had been able to pull a report which showed which of the recalled formulas were in inventory and any patients who had received the recalled product. We further expanded our use of the system in November 2019 when we hired a full-time nutrition lab supply technician who began scanning every case of formula/fortifier upon arrival into the hospital so that the system could be used for inventory management. The goals are to reduce waste by ensuring products are not unnecessarily ordered and that older items are used first to prevent waste from items expiring. The scanning of oral supplement formulas provided by the food services department on the patient’s tray (as opposed to oral supplement formulas provided directly by the Nutrition Lab) was initiated in February 2020. This ensures that no products are distributed to a patient at any level of the organization without scanning to ensure lot numbers are tracked.
CHOC has gained national recognition and is often the benchmark for organizations in building an Infant and Enteral Feeding Safety program. Showing continued improvement and sustainment of the people, process, and technology to keep their infants fed safely and to promote the process of infant feeding HM to their infant population.
Appendix: Presentations, Publications and References
Presentations
Abbott Nutrition Health Institute CE Webinars (2015)
- Regulations, Guidelines, or Mere Suggestions—Standardization of Feedings in the NICU
- Whose Milk is This & What’s in It? Safe Delivery of Infant Feedings in the NICU
Abbott Nutrition Health Institute: Nutrition News You Can Use—From Breastfeeding Best
Practices to Implementing Change (2014, 2015, 2016, 2017, 2018, 2019)
- Infant Feeding Best Practices for Healthcare Facilities
- 15-30 presentations per year between 2014-2019
Academy of Nutrition & Dietetics Annual Food & Nutrition Conference & Expo (FNCE) (2015)
- RDs in Action: Improving Quality Care Using the National Quality Strategy
Academy of Nutrition & Dietetics, Clinical Nutrition Management Annual Symposium (2014)
- Infant Feeding Best Practices
ANHI CE/CME Webinar-U.S. (2018)
- Handling Human Milk and Formula in Healthcare Facilities: Tried & True & What’s New
ANHI CE/CME Webinar-Canada (2018)
- Handling Human Milk and Formula in Healthcare Facilities: Tried & True & What’s New
9th Annual Spectrum of Healthcare from Mother to Child (Texas Perinatal Association) (2017)
- Human Milk and Formula Handling: Best Practices for Healthcare Facilities
Annenberg Center for Health Sciences at Eisenhower CE/CME Webinar (2018)
- Understanding the Current Guidelines for Preparing Pediatric & Newborn Feedings in Health Facilities
Annenberg Center for Health Sciences at Eisenhower Presentation at FNCE (2018)
- Understanding the Current Guidelines for Preparing Pediatric & Newborn Feedings in Health Facilities
Arkansas Academy of Nutrition and Dietetics Annual Meeting (2017)
- Human Milk and Formula Handling: Best Practices for Healthcare Facilities
California Association of Neonatologists—Directors Meeting (2015)
- Centralized Handling & Bar Code Scanning Reduces Breastmilk Errors & Promotes Safety
Children’s Healthcare of Atlanta Clinical Nutrition Education Forum (2015)
- Infant Feeding Best Practices for Children’s Hospitals Human Milk in Feeding Premature
- Infants: From Tradition to Bioengineering in conjunction with the EXPO World’s Fair in Milan, Italy (2015)
- Centralized Handling & Bar Code Scanning of Human Milk Improves Safety
Columbus State Community College Milk Technician Certification Program (2017 and 2018)
- Handling Human Milk and Formula in Healthcare Facilities: Tried & True & What’s New
- Centralized Formula & Human Milk Preparation: Operations Workshop
Dietitians in Nutrition Support & Pediatric Nutrition Practice Group Symposium (2019)
- Human Milk and Formula Handling: Best Practices for Healthcare Facilities
Duke University Medical Center NICU Educational Event (2015)
- Infant Feeding Preparation: Best Practices for Healthcare Facilities
- Preventing Breastmilk Misadministration through Bar Code Scanning
Global Infant Feeding Advisory Board Meeting-Madrid, Spain (2019)
- Human Milk & Formula Handling: Best Practices for Healthcare Facilities (US & Canada)
Health Information Management Systems Society (HIMSS) Annual Conference/Exhibition (2017)
- A Breastmilk Management System Improves Patient Safety
Health Care Compliance Association 20th Annual Compliance Institute (2016)
- Integrating Quality, Compliance, & Clinical Teams: Improved Compliance & Safety
Intermountain Healthcare Annual Conference for Maternal and Infant Medicine (2015)
- Infant Feeding Best Practices for Healthcare Facilities
Medela Education and Clinical Series CE/CME Webinar (2019)
- Safe Handling of Human Milk within Healthcare Facilities
National Association of Neonatal Nurses (NANN) Annual Meeting (2014)
- Infant Feeding Best Practices for Healthcare Facilities
NICU Leadership Forum (2015)
- Improving Safety and Quality with Breastmilk and Formula Handling
NICU Leadership Forum (2014)
- Breastmilk Bar Coding Improves Efficiency for Centralized Breastmilk Preparation
Northern Virginia Academy of Nutrition & Dietetics (2019)
- Infant Feeding Preparation within Healthcare Facilities
Nourishing the Neonate Conference (2014)
- Infant Feeding Best Practices for Healthcare Facilities
Quality and Safety in Children’s Health Conference-Children’s Hospital Association (2016)
- Centralized Breastmilk Handling & Bar Code Scanning Improves Patient Safety
RDs in Practice: Nutrition and Feeding for Infants and Toddlers (2018)
- Best Practices for Handling Human Milk and Formula in Healthcare Facilities
- Human Milk Analysis for the Hospitalized Infant
Safe Handling of Human Milk (Breastfeed LA) (2017)
- Handling Expressed Human Milk and Donor Human Milk in the Hospital Setting
- Starting a Donor Milk Program
Publications
- Bixby, C., Baker-Fox, C., Deming, C., Dhar, V., & Steele, C. (2016). A Multidisciplinary Approach Increases Breastmilk Availability for the VLBW Breastfeeding Medicine, 11(2), 75-79.
- Steele, C. (2018). Best Practices for Handling and Administration of Expressed Human Milk and Donor Human Milk for Hospitalized Preterm Infants. Frontiers in Nutrition, 5(76). doi: 10.3389/fnut.2018.00076
- Steele, C. (2015). Centralized Breast Milk Handling and Bar Code Scanning Improve Patient Safety. In: Human Milk Feeding Premature Infants: From Tradition to Bioengineering. Proceedings of a Consensus Development Conference—EXPO 2015, Milan, Italy. J Ped Gastro Nutr, 61(1), S1-19.
- Steele, C. (2018). Infant Feedings: The Forgotten Patient Safety Risk. Perspectives in Nutrition, 1(1).
- Steele, C. (2020). Safe Handling of Human Milk Within the Hospital Setting. Neonatal Intensive Care. The Journal of Perinatology-Neonatology, 33(3), 11-14.
- Steele, C., Bixby, C. (2014). Centralized Breastmilk Handling and Bar Code Scanning Improve Safety and Reduce Breastmilk Administration Errors. Breastfeeding Medicine, 9(9), 426-429.
- Steele, C., & Collins, E., eds. (2019). Infant and Pediatric Feedings: Guidelines for Preparation of Human Milk & Formula in Health Care Facilities, 3rd Edition. Academy of Nutrition and Dietetics.
- Steele, C., Czerwin, A., Bixby, C. (2015). Breast Milk Bar Code Scanning Results in Time Savings & Staff Efficiency. J Acad Nutr Diet, 115(1), 23-26.
- Steele, C., Ehwerhemuepha, L., & Collins, E. (2020). 24-Hour vs. 12-Hour Storage Recommendations for Previously Frozen (Thawed) Fortified Human Milk. J Acad Nutr Diet, 120(8), 12831287. https://doi.org/10.1016/j.jand.2020.04.017
- Steele, C., Gonzalez, B., & Gornick, W. (2017). Thawing Human Milk for Hospitalized Infants: Use of a Laboratory Bead Bath is an Effective Method. J Acad Nutr Diet. e-publication http://dx.doi.org/10.1016/j.jand.2017.01.024
- Steele, C., & Sullivan, J. (2019) Preparing, Labeling, & Dispensing Enteral Nutrition. In: ASPEN Enteral Nutrition Handbook, 2nd ed.
References
- Steele, C., & Collins, E., eds. (2018). Infant and pediatric feedings: Guidelines for preparation of human milk and formula in health care facilities. 3rd ed. Chicago, IL: Academy of Nutrition and Dietetics. p. 1–248.
- Moro, G.E., Arslanoglu, S., Bertino, E., Corvaglia, L., Montirosso, R., Picaud, J. C., et al. (2015). Human milk in feeding premature infants: From tradition to bioengineering. Proceedings of a consensus development conference—EXPO 2015. J Ped Gastroenterol Nutr, 61, S1–19. doi: 10.1097/MPG.0000000000000897
- Oza-Frank, R., Kachoria, R., Dail, J., Green, J., Walls, K., & McClead, R. E. Jr. (2017). A quality improvement project to decrease human milk errors in the NICU. Pediatrics,139, e2–7. doi: 10.1542/peds.2015-4451
- Steele, C., Czerwin, A., & Bixby, C. (2015). Breast milk bar code scanning results in time savings and staff effi J Acad Nutr Diet, 115, 23–6. doi: 10.1016/j.jand.2014.06.360
- Steele, C., & Bixby, C. (2014). Centralized breastmilk handling and bar code scanning improve safety and reduce breastmilk administration errors. Breastfeed Med, 9, 426–9. doi: 10.1089/bfm.2014.0077
- Barbas, K. H. (2013). Mother’s milk technicians: A new standard of care. J Hum Lact, 29, 323–27. doi: 10.1177/0890334413492910
- Perkey, K. (2016). Delivering results: Opening an infant nutrition center. Future Dimens Clin Nutr Pratc, 8–12.
- National Association of Neonatal Nurses. (2015). The use of human milk and breastfeeding in the neonatal intensive care unit. Position statement #3065. Available online at: http://nann.org/uploads/About/PositionPDFS/1.4.3_Use%20%20of%20Human%20… %20the%20NICU.pdf (Accessed May 3, 2017).
- The Facilities Guidelines Institute. (2014). Guidelines for design and construction of hospital and health care facilities. Washington, DC: American Institute of Architects. p. 91–2. Standard A2.1-7.2.3.2(3) and Standard 2.17.2.3.3(5).
- NSF International Standard/American National Standard for Food Equipment. (2012). Commercial warewashing equipment. Ann Arbor, MI: NSF International.
- NSF International Standard/American National Standard for Food Equipment. (2015). Food equipment materials. Ann Arbor, MI: NSF International.
- Eglash, A., Simon, L., & The Academy of Breastfeeding Medicine. (2017). ABM clinical protocol #8: Human milk storage information for home use for full-term infants, revised 2017. Breastfeed Med, 12, 390–5. doi: 10.1089/bfm.2017.29047.aje
- Boullata, J. I., Carrera, A. L., Harvey, L., Escuro, A. A., Hudson, L., Mays, A., et al. (2017). ASPEN safe practices for enteral nutrition therapy. JPEN J Parenter Enteral Nutr, 41, 15–103. doi: 10.1177/0148607116673053
- The United States Pharmacopeial Convention. (2016). USP 797 pharmaceutical compounding—sterile preparations. In: USP Compounding Compendium 2016. Rockville, MD: The United States Pharmacopeial Convention. p. 39–84.
- The United States Pharmacopeial Convention. (2016). USP 795 pharmaceutical compounding—nonsterile preparations. In: USP Compounding Compendium 2016. Rockville, MD: The United States Pharmacopeial Convention. p. 31–9.
- Jones, F. (2011). Best practice for expressing, storing and handling human milk in hospitals, homes, and child care settings, 3rd Edition. Fort Worth, TX: Human Milk Banking Association of North America, Inc.
- Centers for Disease Control and Prevention. (2017). Hand hygiene in Healthcare Settings. Available online at: https://www.cdc.gov/handhygiene/ providers/index.html (Accessed May 24, 2017).
- Association of Perioperative Registered Nurses. (2021). Hand Hygiene. Available online at: http://www.aorn.org/guidelines/clinical-resources/clinical-faqs/hand-an… (Accessed May 3, 2017).
- Moolenaar, R. L., Crutcher, J. M., San Joaquin, V. H., et al. (2000). A prolonged outbreak of Pseudomonas aeruginosa in a neonatal intensive care unit: Did staff fingernails play a role in disease transmission? Infect Control Hosp Epidemiol, 21, 80–5. doi: 10.1086/501739
- Centers for Disease Control and Prevention. (2002). Guideline for hand hygiene in health-care settings. MMWR Morb Mortal Wkly Rep, 51, 1–56. Available online at: https://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf (Accessed July 16, 2018).
- Hedderwick, S. A., McNeil, S. A., Lyons, M. J., & Kauffman, C. A. (2000). Pathogenic organisms associated with artificial fingernails worn by health care workers. Infect Control Hosp Epidemiol, 21, 505–9. doi: 10.1086/501794
- Yuen, J. W., Loke, A. Y., & Gohel, M. D. I. (2012). Nutritional and immunological characteristics of fresh and refrigerated stored human milk in Hong Kong: A pilot study. Clinica Chimica Acta, 413, 1549–54. doi: 10.1016/j.cca.2012.03.018
- Takci, S., Gulmez, D., Yigit, S., Dogan, O., Dik, K., & Hascelik, G. (2012). Effects of freezing on the bactericidal activity of human milk. J Pediatr Gastroenterol Nutr, 55,146–9. doi: 10.1097/MPG.0b013e31824f7889
- Chang, J. C., Chen, C. H., Fang, L. J., Tsai, C. R., Chang, Y. C., & Wang, T. M. (2013). Influence of prolonged storage process, pasteurization, and heat treatment on biologically-active human milk proteins. Pediatr Neonatol, 54, 360–6. doi: 10.1016/j.pedneo.2013.03.018
- Raoff, A., Adamkin, D. H., Radmacher, P. G., & Telang, S. (2016). Comparison of lactoferrin activity in fresh and stored milk. J Perinatol, 36, 207–9. doi: 10.1038/jp.2015.186
- Grazziotin, M. C., Grazziotin, A. L., Vidal, N. M., Freire, M. H., & DaSilva, R. P. (2016). Analysis of the storage methods for raw human milk from mothers with infants admitted to a neonatal intensive care unit, according to Brazilian regulations. J Hum Lact, 32, 446–54. doi: 10.1177/0890334416647710
- Hamosh, M., Ellis, L. A., Pollock, D. R., Henderson, T. R., & Hamosh, P. (1996). Breastfeeding and the working mother: Effect of time and temperature of short-term storage on proteolysis, lipolysis, and bacterial growth in milk. Pediatrics, 97, 493–8.
- Hurrell, E., Kucerova, E., Loughlin, M., et al. (2009). Neonatal enteral feeding tubes as loci for colonisation by members of the Enterobacteriaceae. BMC Infect Dis, 9, 146. doi: 10.1186/1471-2334-9-146
- American Academy of Pediatrics Committee on Nutrition. (2014). Formula feeding of term infants. In: Kleinman, R. E., Greer, F. R., editors. Pediatric Nutrition, 7th Edition. Elk Grove Village, IL: American Academy of Pediatrics. p. 66–8.
- United States Department of Agriculture. (2015). feeding infants: A guide for use in the child nutrition programs. Available online at:https://www.amazon.com/Feeding-Infants-Guide-Nutrition-Programs/dp/1511….
- Petersen, S., Greisen, G., & Krogfelt, K. (2016). Nasogastric feeding tubes from a neonatal department yield high concentrations of potentially pathogenic bacteria – even 1 day after insertion. Pediatric Res, 80, 395– 400. doi: 10.1038/pr.2016.86
- Perry, J., Stankorb, S., & Salgueiro, M. (2015). Microbial contamination of enteral feeding products in thermoneutral and hyperthermal ICU environments. Nutr Clin Pract, 30, 128–33. doi: 10.1177/0884533614541680
- Takahashi, K., Mizuno, K., & Itabashi, K. (2012). The freeze-thaw process and long intervals after fortification denature human milk fat globules. Am J Perinatol, 29, 283–8. doi: 10.1055/s-0031-1295659
- Choi, A., Fusch, G., Rochow, & Fusch, C. (2016). Target fortification of breast milk: Predicting the final osmolality of the feeds. PLoS ONE, 11, e0148941. doi: 10.1371/journal.pone.0148941
- Kreissl, A., Zwiauer, V., Repa, A., Binder, C., Haninger, N., Jilma, B., et al. (2013). Effect of fortifiers and additional protein on the osmolarity of human milk: Is it still safe for the premature infant? J Pediatr Gastroenterol Nutr, 57, 432–7. doi: 10.1097/MPG.0b013e3182a208c7
- Guenter, P., Hicks, R. W., Simmons, D., Crowley, J., Joseph, S., Croteau, R., et al. (2008). Enteral feeding misconnections: A consortium position statement. Jt Comm J Qual Patient Saf, 34, 285–92. doi: 10.1016/S1553-7250(08) 34035-5
- GEDSA Guidance Supporting ISO 80369-3 ENFit® (2017). Available online at: http://stayconnected.org/wp-content/uploads/2017/11/GEDSA-ENFit-Guidanc… (Accessed July 16, 2018)
The views and opinions expressed in this content or by commenters are those of the author and do not necessarily reflect the official policy or position of HIMSS or its affiliates.



